Figure 2. A: Diameter during BL, min 2 of CPT, and REC. B: Diameter during BL, OCL, and PEAK in OT and OI subjects for FMD and FMD+CPT.
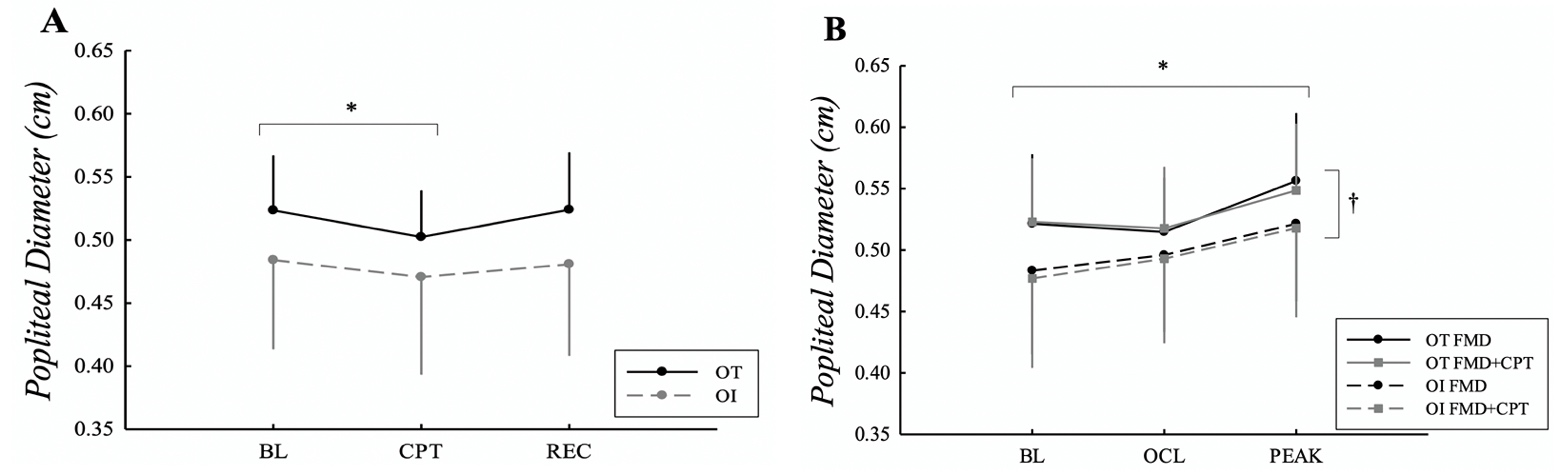
*Difference from BL in both groups. †Difference between groups. P < 0.05.
ENDOTHELIUM DEPENDENT VASODILATION IS NOT ENHANCED IN HEALTHY WOMEN WITH VARYING DEGREES
OF ORTHOSTATIC INTOLERANCE
Alexa M. Brooks, Tinna Traustadóttir, J. Richard Coast and Sara S. Jarvis
Department of Biological Sciences
Northern Arizona University
Submitted March 2020; Accepted in final form June 2020
Brooks AM, et al. The purpose of this study was to determine if the vasodilator capacity of the popliteal artery in women was augmented or the vasoconstrictor capacity in the lower extremity was attenuated in women with orthostatic intolerance. Orthostatic tolerance was assessed using a graded lower body negative pressure test and cumulative stress index (CSI). Popliteal artery diameter and velocity (Doppler ultrasound) was measured in 13 orthostatically tolerant and 7 orthostatically intolerant (OT: CSI range, -633 to -1875 mmHg; OI: CSI range, -80 to -552 mmHg·min; P = 0.015) women after 5 min of distal calf occlusion (FMD), 3 min of cold pressor test (CPT), and 5 min of distal calf occlusion combined with CPT (FMD+CPT). Peak popliteal diameter, measured during FMD+CPT was not different from peak popliteal diameter during FMD. Popliteal FMD, normalized to the shear stimulus, was not different between OT and OI women or between FMD and FMD+CPT. Despite similar vasoconstrictor responses to CPT, assessed by the reduction in peak popliteal artery conductance (OT: -11.0±15.6%; OI: -9.1±23.8%), the magnitude of change in peak popliteal conductance actually increased during FMD+CPT and was similar in OT vs. OI women (OT: 5.8±31.5%; OI: 47.2±60.1%). In conclusion, endothelium dependent vasodilation of the popliteal artery at rest is not enhanced in healthy women with varying degrees of orthostatic intolerance. The increase in popliteal vascular conductance during the combined stimulus (FMD+CPT) may suggest inhibition of sympathetic vasoconstriction via nitric oxide mediated vasodilation (or other dilators) and/or differential control of sympathetic activation in the arms and legs.
Key Words: popliteal artery, flow mediated dilation, lower body negative pressure, endothelial function
INTRODUCTION
It is estimated that about 500,000 Americans suffer from orthostatic intolerance, which is the second most common blood pressure (BP) regulation disorder (Ali et al., 2000). Orthostatic intolerance is the inability to maintain BP in the upright posture and can be experienced by people with autonomic dysfunction, astronauts returning from spaceflight, and subjects post bed rest (Bonnin et al., 2001). However, even in the absence of an underlying cause, healthy individuals and even athletes may experience symptoms of insufficient BP regulation upon assuming the upright position.
In order to maintain an upright posture, it is critical to have an adequate vasoconstrictor response during an orthostatic stress (Montgomery et al., 1977), indicating that the balance between vasoconstriction and vasodilation is crucial in BP regulation. The vascular smooth muscle is responsible for vasoconstriction and vasodilation of the peripheral vasculature and receives input, not only from humoral and neural mechanisms, but also from local agents released by the endothelium. The potential role of the endothelium in BP regulation during standing has not been well studied.
In order to maintain cardiovascular homeostasis, paracrine agents are released by the endothelium, that interact with humoral and neural mechanisms (Guazzi et al., 2005). Endothelial function is assessed by the degree of endothelial-dependent vasodilation which occurs mainly through the nitric oxide (NO) pathway (Green et al., 2014). NO is produced in response to alterations in shear wall stress, which is a major stimulus of the endothelium (Bonnin et al., 2001). Flow mediated dilation (FMD) is a common noninvasive assessment of vascular endothelial function via ultrasound assessment of vessel diameter and blood velocity changes in response to occlusion-induced hyperemia (Harris et al., 2010). An orthostatic challenge is characterized by acute changes in arterial BP and flow, which will increase wall shear stress by pooling of the blood in the dependent regions of the body (Bonnin et al., 2001). This suggests that NO production and release will be stimulated during orthostasis, potentially causing vasodilation if not adequately offset by other vasoconstrictive mechanisms (Goswami et al., 2013).
Studies have suggested that there is augmented endothelial function in patients with neurally mediated syncope, as well as after prolonged periods of head down bed rest (Galetta et al., 2006; Parker et al., 2007; Takase et al., 2000). For instance, seven days of bed rest enhanced FMD in the brachial artery, which was negatively correlated with the duration of the standing test in symptomatic subjects (Bonnin et al., 2001). This suggests that augmented vasodilation contributes to insufficient BP regulation during an orthostatic stress. In a similar case, the FMD response in the anterior tibial artery, post bed rest, was also augmented indicating enhanced endothelium dependent vasodilation after bed rest in both extremities (Platts et al., 2009). Together, these data suggest that there is a relationship in vascular responses between the upper and lower limbs after prolonged head down bed rest.
Not only is the vasodilatory aspect of vascular tone altered in individuals with orthostatic intolerance, there is attenuated vasoconstriction in these populations as well. For example, patients with postural orthostatic tachycardia syndrome, a variant of orthostatic intolerance, do not exhibit normal arterial vasoconstriction in the arteries of the lower extremities and experience increased blood flow to the lower extremities when assuming the upright position (Stewart, 2002). Additionally, there is reduced capacity for vasoconstriction after bed rest and spaceflight (Buckey et al., 1996). Shoemaker et al. (1998) demonstrated that forearm blood flow and vascular conductance decreased in subjects post bed rest and that sympathetically mediated vasoconstriction of the forearm during FMD was attenuated. Accordingly, others (Lind et al., 2002) reported a reduction in brachial FMD during a cold pressor test (CPT), which causes an acute increase in sympathetic tone. By overlaying the CPT during the FMD, the balance between vasodilation and vasoconstriction can be studied. This unique design (Parker et al., 2007) has not been investigated in healthy individuals with varying degrees of orthostatic intolerance.
Orthostatic intolerance tends to affect more women than men (Convertino, 1998; Easton et al., 2009) and one explanation includes the influence of estrogen on the peripheral vasculature. For example, estrogen decreases vascular tone via the NO pathway (Waters et al., 2002). Additionally, women have demonstrated blunted responsiveness to vasoconstrictors including norepinephrine and endothelin (Ergul et al., 1998; Kneale et al., 2000). Considering this evidence, women demonstrate decreases in vascular tone and blunted responses to vasoconstrictors, which could contribute to their increased susceptibility to orthostatic intolerance. Thus, the purpose of this study was to determine if there is enhanced endothelium dependent vasodilation of the popliteal artery or if there is decreased ability to vasoconstrict a dilated vessel in the dependent region (a region important for the maintenance of upright blood pressure) in those with orthostatic intolerance when compared to control group. We hypothesized that women with orthostatic intolerance would have enhanced endothelium dependent vasodilation compared to control women, as seen as augmented popliteal FMD responses. It was further hypothesized that women with orthostatic intolerance would have attenuated popliteal vasoconstrictor responses to the CPT during FMD.
METHODS
Participants
Sample size determination was performed in SigmaPlot 12.5 (San Jose, CA). Based on Takase et al. (2000), who examined those with neurally mediated syncope and controls, it was determined that 12 subjects were needed in each group—orthostatically tolerant (OT) vs. orthostatically intolerant (OI) to detect a difference of 6.0% in FMD between groups with a SD of 5.0% (power of 80% and alpha set at 0.05). Twenty-eight subjects volunteered and gave their written informed consent to participate in the study, which was approved by the Northern Arizona University Institutional Review Board. Eight women were excluded due to the following reasons: one woman had unusable data, one woman did not follow pre-study instructions outlined in the experimental design, and the five other women due to scheduling conflicts. Subjects were divided into OT (13 subjects) and OI (7 subjects). Orthostatic intolerance was defined as a cumulative stress index (CSI) ≥ -600 mmHg*min as measured in the lower body negative pressure test described below (Wenner et al., 2013) . Baseline subject characteristics are outlined in Table 1. Exclusionary criteria were: smoking or living with a smoker, excessive alcohol consumption (>2 alcoholic beverages, 3x/week), recreational drug use, significant medical history including cardiovascular, pulmonary, and metabolic diseases, use of medications with hemodynamic effects, fainting disorders, body mass index ≥ 30 kg/m2, systolic blood pressure (SBP) < 90 mmHg, blood donation within last six months, or current pregnancy. All subjects reported normal menstrual cycles.
Subjects underwent a screening protocol, which consisted of a 12-lead resting electrocardiogram and BP measurement, evaluation of medical history, and completion of the International Physical Activity Questionnaire (Craig et al., 2003).
Protocol
Subjects reported to the Cardiovascular Regulation Laboratory at Northern Arizona University for the study visit following a 4-hr fast, having refrained from vitamin supplementation for 72-hrs prior to testing, refrained from caffeine and alcohol for 48-hrs prior to testing, and limited physical activity
Table 1. Subject Characteristics.
|
|
Tolerant (n=13) |
Intolerant (n=7) |
|
Age (yrs) |
20.8±1.5 |
21.4±3.1 |
|
Height (m) |
1.65±0.06 |
1.63±0.05 |
|
Weight (kg) |
62.8±9.1 |
57.8±8.2 |
|
BMI (kg/m2) |
22.8±2.8 |
21.7±2.2 |
|
BSA (m2) |
1.7±0.1 |
1.6±0.1 |
|
SBP (mmHg) |
108±7 |
108±6 |
|
DBP (mmHg) |
72±5 |
72±7 |
|
HR (bpm) |
70±9 |
79±9 |
|
Total MET-min/week |
3921±3070 |
5176±1454 |
|
Total Sitting min/day |
425.6±161.2 |
265.7±116.0 |
|
CSI (mmHg·min) |
-923.4±686.4 |
-334.8±220.9* |
Data are expressed as means±SD. BMI, body mass index; BSA, body surface area; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; CSI, cumulative stress index. *Significant difference between groups. P < 0.05.
for 12-hrs prior. Subjects were instructed to empty their bladders and a pregnancy test was administered. A negative test was required before testing. Participants were tested during the early follicular phase of the menstrual cycle to minimize the influence of female hormones. The subject’s weight was obtained at the beginning of the study visit.
All subjects participated in 4 parts of the experiment (Figure 1) in a single study visit in which Doppler ultrasound was used to assess popliteal diameters and velocities. The 4 parts included FMD, CPT, and CPT superimposed on FMD (FMD + CPT), as well as a graded maximal lower body negative pressure (LBNP) test used to determine CSI and assess orthostatic tolerance. Each part of the experiment was separated by a 10-min rest period. FMD, CPT, and FMD + CPT were performed in a randomized order, while LBNP was always performed last. Beat-by-beat BP (Finometer MIDI, ADInstruments, Colorado Springs, CO) and HR (ECG module, ADInstruments, Colorado Springs, CO) were continuously measured throughout the study visit. BP was also obtained via electrosphygmomanometry (SunTech Tango+, Morrisville, NC) on the brachial artery. Popliteal diameter and velocity were measured during FMD, CPT, and FMD + CPT using ultrasound. The artery was imaged with a 5-8 MHz multifrequency linear array probe (Acuson Sequoia C256, Acuson Corporations, Mountain View, CA). Once a satisfactory image was obtained using B-mode imaging, the placement of the probe was marked on the leg to ensure the site of measurement did not change during the experiment. Doppler velocity was measured using the standard 60˚ angle of insonation (Harris et al., 2010). The ultrasound protocols were recorded on videotape for subsequent off-line analysis. All sessions were conducted in dimmed light with an ambient temperature of 22.1±2.2°C.
Figure 1. Timeline of experimental protocol.
|
FMD |
Break (10 min) |
CPT |
Break (10 min) |
FMD + CPT |
Break (10 min) |
LBNP |
|
Tests (FMD, CPT or FMD+CPT) in randomized order Diameter, velocity, BP and HR continuously measured |
BP and HR |
|||||
FMD, flow mediated dilation; CPT, cold pressor test; LBNP, lower body negative pressure; BP, blood pressure; HR, heart rate.
Popliteal FMD was measured in the left leg with the subject lying prone. A BP cuff was placed around the calf, 5-8 cm distal to the popliteal fossa. After the subject rested in the prone position for at least 10 min, resting popliteal artery diameter and velocity were measured for 1 min before inflation of the cuff. The cuff was inflated to ~250 mmHg for 5 min; diameter and velocity recordings resumed 1 min before cuff deflation and continued for 5 min after deflation. For the CPT, baseline (BL) measurements of diameter and velocity were taken for 1 min. Two bags of a 2-4˚C mixture of ice and water were placed on the right hand (one bag on bottom and one bag on top) for 3 min. Diameters and velocities were measured throughout the CPT, and measurements continued for 3 min of recovery after the bags were removed from the subject’s hand. For assessment of FMD with the CPT, BL diameter and velocity measurements were taken for 1 min. The FMD protocol was repeated in an identical fashion except that CPT (as described above) was superimposed on the protocol such that the ice water bags were placed at minute 3.5 of the 5-min occlusion (OCL) and continued for 3 min until 1.5 min after cuff release. This timing ensured that a sufficient sympathetic stimulus would occur during peak vasodilation. For the LBNP procedure, subjects laid in the supine position with their lower body inside the LBNP box, which was sealed at the level of the iliac crest. After ~20 min of supine rest, 5 min of BL measurements commenced. The LBNP test started with the application of negative pressure at -10 mmHg for 5 min, followed by -15 mmHg for 5 min. Each subsequent stage decreased pressure by 5 mmHg (-20, -25, -30, -35, -40 mmHg) in 5 min intervals until presyncope. If the subject reached -40 mmHg, the pressure would remain at -40 mmHg until presyncope or 60 min of LBNP. The CSI was calculated for each subject by summing the product of the negative pressure (mmHg) and the time (min) spent at that stage (Wenner et al., 2013). A more negative CSI indicated a more negative pressure attained prior to presyncopal symptoms and thus greater orthostatic tolerance. The subjects were then divided into orthostatic tolerant (OT) and orthostatic intolerant (OI) groups.
Beat-by-beat BP was monitored and test termination was determined using any one of the following criteria: a decline in SBP >20 mmHg; a decrease in SBP to <70 mmHg; a decrease in SBP to <90 mmHg associated with symptoms of dizziness, light-headedness, nausea, difficulty breathing, sweating, or a loss of peripheral vision; a rapid decline in HR of >25 bpm; or progressive symptoms of presyncope accompanied by a request from the subject to terminate the test. After LBNP was terminated and chamber pressure returned to atmospheric pressure, subjects remained supine for a 5 min recovery.
During data analysis, the popliteal artery diameter and velocity measurements for FMD measurements were obtained for 1 min at BL and during the last minute of OCL. BL and OCL diameters were calculated as the average of 10 consecutive cardiac cycles taken over the 1-min BL and last minute of OCL, respectively (Harris et al., 2010). Measurements of diameter were also taken at 40, 50, 60, 70, 80, 90, 100, 110, 120, 180, 240, and 300 s after cuff deflation. Three consecutive cardiac cycles were analyzed for post-deflation time points (Liang et al., 1998). Measurements at each cardiac cycle were sampled at end diastole and averaged from 3 measurements taken from anterior to posterior wall (Harris et al., 2010). Blood flow velocity was measured at BL, during the last minute of OCL, and 30 s after cuff release. A time-averaged, angle-corrected maximum velocity (highest velocity across the cardiac cycle) was calculated from a trace of the velocity-time integral from the average of 10 full cardiac cycles for BL and OCL velocities (Harris et al., 2010).
FMD was calculated as the absolute and percent change in diameter from either BL or the last minute of OCL to peak. We calculated post OCL area under the curve (AUC) shear rate (SR, 4*velocity/diameter) with the trapezoidal rule, using the diameter measured immediately before cuff release (OCL diameter) and velocity measurements for 30 s after cuff release (until it was necessary to switch into high-resolution 2D mode to image peak diameters). Previous research (Parker et al., 2006) demonstrated that diameter does not change appreciably after cuff release when peak blood velocity and shear rate are observed, and the Sequoia ultrasound machine is not able to measure high-resolution diameters and velocity simultaneously. FMD was then normalized to the 30 s AUC SR, which has a good correlation between the SR calculated to 60 s or to the individual peak diameter (Thijssen et al., 2011). FMD is reported as the peak %change in diameter (%FMD) from the OCL measurement. This measurement was used as adjustments to the image were sometimes necessary during OCL and thus the probe position and image during the OCL were the same as those during the release period.
BL, OCL, and peak popliteal blood flow (PBF) were derived from the formula: blood velocity•π•(popliteal diameter/2)2•60; where PBF is in mL/min, blood velocity is in m/s, popliteal diameter is in mm, and 60 is used to convert from mL/s to mL/min. Resting, OCL, and peak popliteal vascular conductance (PVC) were calculated as PBF/mean arterial pressure (MAP).
To examine the influence of CPT on peak reactive hyperemia, we calculated the percent reduction in hyperemic PVC during sympathetic stimulation as: (peak PVCFMD+CPT – Peak PVCFMD)/Peak PVCFMD • 100; where the difference between PVC measured after 5-min calf OCL and PVC measured when CPT was superimposed on OCL is divided by PVC measured after 5-min calf OCL. Additionally, we compared this value with the PVC percent reduction relative to rest observed during CPT alone (PVCrest – PVCCPT)/PVCrest * 100) to account for any differences in vasoconstrictor responses.
To examine the influence of CPT on FMD, we examined all components of the dilatory response– absolute diameter changes, percent diameter changes relative to BL or OCL, 30-s AUC SR, and percent diameter changes normalized to the AUC SR-between FMD and FMD+CPT.
Statistical Analysis
Data are reported as means ± SD. Statistical analysis was carried out using a t-test to assess BL subject characteristics. Mann-Whitney Rank Sum test was used if normality failed. A three-way ANOVA was used to compare diameter, blood flow, and vascular conductance in FMD and FMD+CPT [group (OT, OI) x condition (FMD, FMD+CPT) x stage)], and a two way repeated measures ANOVA was used to compare BP, diameter, blood flow, and vascular conductance in CPT (group x stage), and to compare %dilation and changes in conductance between groups and conditions (group x condition). Tukey’s post hoc analysis was used when significance was found. Statistical analyses were performed using Sigma Plot (San Jose, CA). The LIFEREG procedure in SAS 9.4 (Cary, NC) was used to assess the relationship between FMD and cumulative stress index. LIFEREG allows for regression analysis to be performed on right censored data, since we terminated lower body negative pressure at 60 min, if individuals did not become presyncopal. In all cases, differences with P < 0.05 were considered significant.
RESULTS
Baseline subject characteristics are outlined in Table 1. Subjects were well matched for baseline characteristics; there were no significant differences between the women in the OT and OI groups in age, height, weight, BMI, body surface area (BSA), SBP, diastolic blood pressure (DBP), HR, total MET-min/week, or total sitting min/day. CSI was significantly different between OT and OI subjects (OT: range, -633 to -1875 mmHg; OI: range, -80 to -552 mmHg·min; P = 0.015).
Absolute diameter responses between FMD and FMD+CPT are shown in Fig. 2. Popliteal diameter was smaller in OI women compared to OT women during FMD and FMD+CPT (OT: 0.53±0.05 cm, OI:
Table 2. BP and HR during CPT.
|
|
|
OT |
OI |
|
SBP (mmHg) |
BL |
109±8 |
103±7 |
|
CPT |
114±11* |
107±7* |
|
|
REC |
110±10 |
103±5 |
|
|
DBP (mmHg) |
BL |
68±7 |
70±6 |
|
CPT |
77±8* |
72±7 |
|
|
REC |
69±8 |
68±9 |
|
|
HR (bpm) |
BL |
69±8 |
70±11 |
|
CPT |
74±9* |
74±15* |
|
|
REC |
68±7 |
69±13 |
*Difference from BL. P < 0.001
0.50±0.07 cm, P = 0.004), but was similar between conditions. Diameter increased after cuff release compared to BL (Fig. 2B) and was similar between conditions and groups (OT FMD: BL, 0.53±0.06 cm; Peak, 0.54±0.06 cm; P = 0.023), and was decreased during CPT compared to BL in both groups (OT: BL, 0.53±0.04 cm; CPT, 0.51±0.04 cm; P < 0.001). SBP and DBP increased similarly during CPT compared to BL in both groups (OT SBP: BL, 108±8 mmHg; CPT, 114±11 mmHg; OT DBP: BL, 68±7 mmHg; CPT, 77±8 mmHg; both P < 0.001). The OCL dilatory response normalized to the AUC SR was not significantly different between the OI and OT groups or between conditions (Fig. 3).
Figure 2. A: Diameter during BL, min 2 of CPT, and REC. B: Diameter during BL, OCL, and PEAK in OT and OI subjects for FMD and FMD+CPT.

*Difference from BL in both groups. †Difference between groups. P < 0.05.
Figure 3. Comparison of
average normalized FMD responses during FMD and FMD+CPT in OT and OI groups.
Dilation was calculated as %increase above OCL diameter divided by the 30-s AUC
for shear rate immediately following cuff release.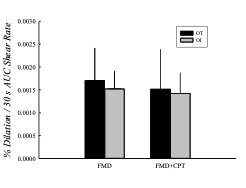
Blood pressure and HR responses to CPT are outlined in Table 2. SBP was similar between OT and OI subjects and increased during CPT compared to BL (P < 0.001). DBP increased during CPT compared to BL in OT subjects (P < 0.001), but was similar between stages in OT subjects. HR increased during CPT in both groups compared to BL (P < 0.001).
Popliteal BF and VC are outlined in Table 3. BF was not different between the groups, and there was an increase during PEAK compared to BL and OCL during FMD and FMD+CPT (P < 0.001). BF was similar between FMD+CPT and FMD in both groups (P = 0.05). There was no difference in BF during CPT between groups or compared to BL. VC was similar between groups, and was larger during PEAK compared to BL and OCL during FMD and FMD+CPT (P < 0.001), and smaller during CPT (P = 0.025).
Percent changes in conductance were similar between OT and OI subjects (Fig 4). Both groups had a similar reduction in vascular conductance from BL to CPT, however both groups saw an increase in conductance after superimposition of CPT on FMD compared to FMD (OT: CPT, -11.0±15.6%, FMD+CPT, 5.8±31.4%; OI: CPT, -9.1±23.8%, FMD+CPT, 47.2±60.0%; CPT to FMD+CPT, P = 0.004). Regression analysis revealed that there was no relationship between FMD and CSI (P = 0.78).
Table 3. Popliteal blood flow and vascular conductance during FMD, FMD+CPT, and CPT at BL, OCL, PEAK, and CPT in tolerant and intolerant subjects.
*Difference from BL and OCL in FMD and FMD+CPT in both groups. **Difference from BL in CPT in both groups. P < 0.05
Figure 4. Resting % reduction in conductance during CPT. % change in peak popliteal (post-hyperemic) conductance when CPT was superimposed on FMD (CPT+FMD).
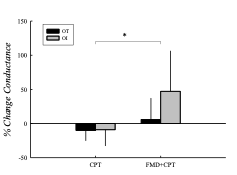 *Difference between conditions. P < 0.05.
*Difference between conditions. P < 0.05.
DISCUSSION
Evidence suggests that there is an imbalance between vasodilation and vasoconstriction in patients with orthostatic intolerance. This alteration in vessel tone has not been characterized in the healthy population. The present study utilized FMD and concomitant application of the sympathoexcitatory CPT in healthy orthostatically tolerant and intolerant subjects to test the hypothesis that endothelial function would mediate orthostatic tolerance. The major findings of this study were: 1) there was no difference in popliteal artery FMD between OT and OI subjects, 2) both groups demonstrated a larger peak conductance when CPT was superimposed on FMD compared to FMD alone, and 3) changes in popliteal vascular conductance during CPT and FMD+CPT were similar between OT and OI subjects. These findings do not support the hypothesis that individuals with orthostatic intolerance have augmented popliteal FMD compared to tolerant individuals, nor that intolerant subjects have attenuated vasoconstriction during the CPT.
We demonstrated that FMD was not different between groups and that there was no relationship between FMD and CSI. Previously reported, the flow induced dilation translates to 5%-15% of the BL diameter in healthy individuals (De Roos et al., 2003). Both groups in the current study fell within this healthy range and the intolerant group did not have augmented responses as expected. We hypothesized that popliteal FMD would be enhanced in orthostatically intolerant subjects because it has been previously shown that anterior tibial artery FMD was augmented post bed rest in Platts et al. (2009). In another case, Bonnin et al. (2001) reported enhanced FMD following bed rest that was negatively correlated with the duration of the standing test in symptomatic subjects. This suggests that the endothelium dependent vasodilatory pathway may have been enhanced after bed rest induced orthostatic intolerance. To illustrate this point, research has reported that acute hind-limb unweighted rats experienced an upregulation of the endothelial-dependent NO/cGMP pathway through NO synthase phosphorylation (Vaziri et al., 2000; White et al., 2010).
However, this alteration in endothelial function may not translate to a healthy population. Prior to bed rest, Bonnin et al. (2001) reported that there was no correlation between FMD and tolerance to the standing test. Physical inactivity during bed rest represents a strong stimulus that induces remodeling of artery diameter, specifically decreases in the femoral artery (van Duijnhoven et al., 2010). It is proposed that this arterial remodeling leads to the orthostatic intolerance seen after durations of physical inactivity that occur in bed rest and space flight. We found that our subjects were well matched for physical activity. The IPAQ revealed that OI subjects were not less physically active than OT subjects and did not have increased weekly sitting time, which indicates that physical inactivity and arterial remodeling did not contribute to the orthostatic intolerance in our subjects. Even though OI subjects had decreased popliteal diameters compared to OT subjects in the FMD and FMD+CPT tests, their percent dilations were not larger. It has been reported that FMD shows an inverse correlation with increasing vessel size; however, our results did not demonstrate a similar correlation (Celermajer et al., 1992; Schroeder et al., 2000).
Another explanation contributing to decreased orthostatic tolerance could involve inadequate baroreflex mediated vasoconstriction or attenuated release of endothelin upon upright posture. For example, women have demonstrated blunted responsiveness to vasoconstrictors including norepinephrine and endothelin (Ergul et al., 1998; Kneale et al., 2000). Baroreflex-mediated increases in peripheral vasoconstriction and HR are principal mechanisms that defend BP during orthostatic challenges (Spaak et al., 2005). There is, however, enhanced endothelial activity as a physiologic response to upright displacement in human beings, seen as increases in brachial FMD during orthostatic stress (Guazzi et al., 2005; Guazzi et al., 2004). There is the possibility that we did not see an augmented endothelial response because we assessed endothelial function before orthostatic stress rather than during or after. However, based on the current findings, it is likely that the NO mediated vasodilation at rest in the arterial circulation of the lower limbs does not contribute to orthostatic intolerance in a healthy population.
It may also be of importance to note that FMD may be more related to initial orthostatic hypotension rather than total time to presyncope. Initial orthostatic hypotension is associated with an immediate and transient drop in BP due to a large decrease in resistance (similar to the local effects of FMD), while time to syncope is more related to vasoconstrictor reserve and cerebral vasoconstriction (Thomas et al., 2009). For example, Fu et al. (2005) concluded that a person’s orthostatic tolerance is greatly dependent upon their vasoconstrictor reserve during posture change. More specifically, women have increased compliance in capacitance vessels in the pelvic area, which creates more pooling of blood in the pelvic region during orthostasis, leading to reduced preload and decreased tolerance (Fu et al., 2005; Jarvis et al., 2011). The mobilization of blood volume is mainly limited to the veins of the splanchnic circulation because, unlike the veins in the legs, it is more compliant and is richly innervated with sympathetic nerves (Gelman, 2008). Therefore, it is likely that regional differences might exist and attenuated vasoconstriction and increased pooling may be more likely to occur in areas such as the splanchnic vasculature of women, compared to the arteries in the lower legs.
During FMD+CPT, there was an increase in peak conductance compared to FMD alone. These results differ from previous findings of a 5-8% reduction during superimposition of CPT on FMD relative to FMD alone (Parker et al., 2007). However, another study reported that acute stimulation of the sympathetic nervous system using the CPT decreased FMD in the arm, but did not alter the FMD response of the superficial femoral artery (Thijssen et al., 2006). Additionally, Jacob et al. (2000) reported that norepinephrine spillover increased similarly in both arms and legs during the CPT; however, vascular resistance increased in the arms but unexpectedly decreased in the legs (Jacob et al., 2000). This suggests that limb differences in adrenergic receptor sensitivity or density may account for different FMD responses of the upper and lower limbs during acute sympathetic stimulation.
The measure of FMD in brachial arteries cannot be extrapolated to the lower limb vessels (Thijssen et al., 2011b). Thijssen et al. (2011b) showed that there was no correlation between FMD in the conduit arteries of the upper limb (brachial) and in the lower limbs (superficial femoral and popliteal) in healthy young men. These differences in vascular responsiveness between the arm and the leg have been characterized in previous work, demonstrating that lower limb arteries have decreased shear rate and decreased FMD (Newcomer et al., 2004; Newcomer et al,, 2008). However, Nishiyama et al. (2007) reported that the brachial artery tended to have a greater FMD than the popliteal artery when expressed in %dilation above BL diameter, in contrast to a significantly greater FMD in the popliteal artery when %dilation was matched for shear stimulus. These two vessels could possibly have differing shear stress sensitivities resulting in enhanced vasodilation in the leg (Nishiyama et al., 2007).
Padilla et al. (2009) hypothesized that there is a limb-specific response to increased hydrostatic pressure. They found that brachial artery FMD was impaired after a bout of increased hydrostatic pressure imposed by arm hanging (~3.5% reduction in brachial FMD), however popliteal artery FMD was unaltered after a short episode of upright sitting (~1.5% reduction in popliteal FMD) which increased the pressure gradient in the legs to a greater degree than the arm hanging (Padilla et al., 2009). These data suggest lesser sympathetic control of the arterial circulation of legs compared with arms in humans (Jacob et al., 2000) or differences in receptor density or sensitivity caused from chronic exposure to larger arterial and hydrostatic gradients.
There was no difference in the decrease in conductance (~9%) seen during CPT between groups, which indicates that each group had similar vasoconstrictor responses, however this decrease in conductance was much lower than previously reported (~27%) (Parker et al., 2007). A potential explanation may be that the CPT stimulus was not adequate to see the expected sympathetic response, which may also be a reason for the increase in conductance during FMD+CPT. When comparing BP responses to previous research using an ice water slurry, the methods in the current study did not produce comparable BP responses. In previous research in our lab (unpublished findings), healthy young women had an increase in SBP of 24±12 mmHg (range: 7 – 42 mmHg) and in DBP of 20±8 mmHg (range: 4-32 mmHg). The subjects in the current study had an increase in SBP of 5±6 mmHg (range: -3 – 20 mmHg) and in DBP of 6±8 (range: -1 – 25 mmHg). Others have also reported that healthy young women had an increase in SBP of 13±2 mmHg and in DBP by 11±1 mmHg in response to the CPT using ice water slurry (Greaney et al., 2015). Other researchers have shown that the modality of cold application has an effect on cooling efficiency, or the ability of the cooling agent to bring the skin temperature to homeostasis (Kennet et al., 2007). Since the ice water was contained in sealed bags and not in direct contact with the skin, it is possible that there was diminished heat transfer from the subjects. This could decrease the stimulus of cold and pain needed to sympathetically increase vasoconstriction and BP. The experimental setup was chosen because subjects were required to lay prone on the experimental table in a position that allowed adequate imaging of the left leg with the ultrasound machine. This did not allow enough room for the right arm to hang over the side of the table and be placed in an ice water slurry.
Sympathetic activation is not homogenous and depending on the stimuli, may induce preferential vasoconstriction in some vascular beds and not in others (Jacob et al., 2000). An enhanced vasodilator response during sympathetic stimulation has been previously reported (Harris et al., 2000). A 64% average increase in brachial FMD has been reported during mental stress (Harris et al., 2000). A potential explanation may be that the catecholamines released during a mental stress test enhance the bioavailability or augment the release of NO and override the vasoconstrictor effect of catecholamines. Endogenous NO has an inhibitory effect on the stimulated release of catecholamines from the sympathetic nerves possibly due to the direct chemical interaction with the catecholamine as it is released (Kolo et al., 2004). The inhibition of peripheral vasoconstriction has been proposed as an important mechanism of NO mediated vasodilation (Guazzi et al., 2005).
There are a few limitations to this study. One, the manual assessment of ultrasound parameters was a limitation. This method is highly operator
dependent and subject to observer error (Thijssen et al., 2011). Computer-assisted analysis that utilizes edge-detection and wall-tracking software has demonstrated significantly lower intra-observer variation compared to the manual technique (Thijssen et al., 2011?) but the former was not available for the present study. Two, the LBNP protocol may have been a limitation. We speculate that the CSI’s from this protocol do not provide an accurate representation of true orthostatic tolerance. Even though we were able to produce the same CSI’s previously stated in other studies between OT and OI subjects, the gradual and low decrease in pressure in our LBNP protocol may not have produced adequate and pronounced pooling of the blood in order to properly distinguish the groups. The degree of pooling is proportional to the negative pressure reached during LBNP (Lathers & Charles, 1993). It was reported that -50 mmHg of LBNP produced changes similar to that of 70˚ head up tilt. Lightfoot and Tsintgiras (1995) found that maximal negative pressure is better at assessing orthostatic tolerance vs time during stages (Lightfoot & Tsintgiras, 1995). Third, the CPT stimulus may not have been adequate to produce a sufficient sympathetic response, which may explain the increase in conductance seen during FMD+CPT. An ice water slurry that allows direct contact of the ice and skin may be needed for greater activation of the sympathetic nervous system. Lastly, the sample size was a limitation. Based on our sample size calculation, 12 subjects were required for each group. We had 13 OT and 7 OI participants. We had 28 subjects give their written informed consent to participate, however 8 of those subjects had to be excluded for reasons mentioned previously.
CONCLUSION
In conclusion, this study examined endothelial responses to FMD, CPT, and FMD+CPT in both OT and OI subjects. Interestingly, we found that resting popliteal artery FMD and changes in vascular conductance were not different between the groups. This suggests that healthy orthostatic intolerant subjects do not have enhanced endothelium dependent vasodilation in the popliteal artery prior to orthostatic stress. Other regions of the body, such as the splanchnic circulation, may play a larger role in BP regulation in orthostatically intolerant women (Fu et al., 2005; Jarvis et al., 2011). The current study reported increased vascular conductance during FMD+CPT compared to FMD, which could be explained by insufficient sympathetic stimulus during the CPT, an inhibition of sympathetic vasoconstriction via NO mediated vasodilation, or differential control of sympathetic activation in the arms vs legs. Causes of orthostatic intolerance are multifactorial and future research is needed to clarify such mechanisms. The recognition of the balance between vasoconstriction and vasodilation is important in the etiology of this condition; however, enhanced vasodilation in the lower extremities at rest of healthy young women does not seem to be a contributing factor.
ACKNOWLEDGEMENT
The Cardiovascular Regulation Laboratory would like to thank the participants for donating their time to the study. We would also like to thank the following undergraduate students for their assistance with the studies: James Brand, Christina Bankemper, Kat Chancey, Geovanni Chavez-Pardini, Andrea Fimbres, Shannon McClain, Macklin McBride, and Eddie Smith.
ADSASDF
Funding
No funding declared to complete this research
REFERENCES
Ali, Y. S., Daamen, N., Jacob, G., Jordan, J., Shannon, J.
R., Biaggioni, I., & Robertson, D. (2000). Orthostatic intolerance: a disorder of young women. Obstet Gynecol Surv, 55(4), 251-259. doi: 10.1097/00006254-200004000-00025
Bonnin, P., Ben Driss, A., Benessiano, J., Maillet, A.,
Pavy le Traon, A., & Levy, B. I. (2001). Enhanced flow-dependent vasodilatation after bed rest, a possible mechanism for orthostatic intolerance in humans. Eur J Appl Physiol, 85(5), 420-426. doi:10.1007/s004210100483
Buckey, J. C., Jr., Lane, L. D., Levine, B. D.,
Watenpaugh, D. E., Wright, S. J., Moore, W. E., Moore, F. A. G., Blomqvist, C. G. (1996). Orthostatic intolerance after spaceflight. J Appl Physiol, 81(1), 7-18. doi: 10.1152/jappl.1996.81.1.7
Celermajer, D. S., Sorensen, K. E., Gooch, V. M.,
Spiegelhalter, D. J., Miller, O. I., Sullivan, I. D., Lloyd, J. K., Deanfield, J. E. (1992). Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet, 340(8828), 1111-1115. doi: 10.1016/0140-6736(92)93147-f
Convertino, V. A. (1998). Gender differences in
autonomic functions associated with blood pressure regulation. Am J Physiol, 275(6 Pt 2), R1909-R1920. doi: 10.1152/ajpregu.1998.275.6.R1909.
Craig, C. L., Marshall, A. L., Sjostrom, M., Bauman, A.
E., Booth, M. L., Ainsworth, B. E., Pratt, M,. Ekelund, U., Yngve, Agneta., Sallis, J. F., Oja, P. (2003). International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc, 35(8), 1381-1395. doi:10.1249/01.MSS.0000078924.61453.FB
De Roos, N. M., Bots, M. L., Schouten, E. G., & Katan,
M. B. (2003). Within-subject variability of flow-mediated vasodilation of the brachial artery in healthy men and women: implications for experimental studies. Ultrasound Med Biol, 29(3), 401-406. doi:10.1016/s0301-5629(02)00709-3
Easton, C., Calder, A., Prior, F., Dobinson, S., I'Anson,
R., MacGregor, R., Mohammad, Y., Kingsmore, David., Pitsiladis, Y. P. (2009). The effects of a novel "fluid loading" strategy on cardiovascular and haematological responses to orthostatic stress. Eur J Appl Physiol, 105(6), 899-908. doi:10.1007/s00421-008-0976-3
Ergul, A., Shoemaker, K., Puett, D., & Tackett, R. L.
(1998). Gender differences in the expression of endothelin receptors in human saphenous veins in vitro. J Pharmacol Exp Ther, 285(2), 511-517. Retrieved from PM:9580591
Fu, Q., Witkowski, S., Okazaki, K., & Levine, B. D.
(2005). Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am J Physiol Regul Integr Comp Physiol, 289(1), R109-R116. doi: 10.1152/ajpregu.00013.2005
Galetta, F., Franzoni, F., Plantinga, Y., Ghiadoni, L.,
Merico, G., Tocchini, L., Braccini, L., Rossi, M., Carpi, A., Taddei, S., Santoro, G. (2006). Endothelial function in young subjects with vaso-vagal syncope. Biomed Pharmacother, 60(8), 448-452. doi:10.1016/j.biopha.2006.07.014
Gelman, S. (2008). Venous function and central
venous pressure: a physiologic story. Anesthesiology, 108(4), 735-748. doi: 10.1097/ALN.0b013e3181672607
Goswami, N., Gorur, P., Pilsl, U., Anyaehie, B., Green,
D. A., Bondarenko, A. I., Roessler, A., Hinghofer-Szalkay, H. G. (2013). Effect of orthostasis on endothelial function: a gender comparative study. PLoS One, 8(8), e71655. doi:10.1371/journal.pone.0071655
Greaney, J. L., Matthews, E. L., & Wenner, M. M.
(2015). Sympathetic reactivity in young women with a family history of hypertension. Am J Physiol Heart Circ Physiol, 308(8), H816-822. doi:10.1152/ajpheart.00867.2014
Green, D. J., Dawson, E. A., Groenewoud, H. M., Jones,
H., & Thijssen, D. H. (2014). Is flow-mediated dilation nitric oxide mediated?: A meta-analysis. Hypertension, 63(2), 376-382. doi:10.1161/HYPERTENSIONAHA.113.02044
Guazzi, M., Lenatti, L., Tumminello, G., & Guazzi, M.
D. (2005). Effects of orthostatic stress on forearm endothelial function in normal subjects and in patients with hypertension, diabetes, or both diseases. Am J Hypertens, 18(7), 986-994. doi:10.1016/j.amjhyper.2005.02.018
Guazzi, M., Lenatti, L., Tumminello, G., Puppa, S.,
Fiorentini, C., & Guazzi, M. D. (2004). The behaviour of the flow-mediated brachial artery vasodilatation during orthostatic stress in normal man. Acta Physiol Scand, 182(4), 353-360. doi:10.1111/j.1365-201X.2004.01365.x
Harris, C. W., Edwards, J. L., Baruch, A., Riley, W. A.,
Pusser, B. E., Rejeski, W. J., & Herrington, D. M. (2000). Effects of mental stress on brachial artery flow-mediated vasodilation in healthy normal individuals. Am Heart J, 139(3), 405-411. doi: 10.1016/s0002-8703(00)90083-8
Harris, R. A., Nishiyama, S. K., Wray, D. W., &
Richardson, R. S. (2010). Ultrasound assessment of flow-mediated dilation. Hypertension, 55(5), 1075-1085. doi:10.1161/HYPERTENSIONAHA.110.150821
Jacob, G., Costa, F., Shannon, J., Robertson, D., &
Biaggioni, I. (2000). Dissociation between neural and vascular responses to sympathetic stimulation : contribution of local adrenergic receptor function. Hypertension, 35(1 Pt 1), 76-81. doi:10.1161/01.hyp.35.1.76
Jarvis, S. S., VanGundy, T. B., Galbreath, M. M.,
Shibata, S., Okazaki, K., Reelick, M. F., Levine, B. D, Fu, Q. (2011). Sex differences in the modulation of vasomotor sympathetic outflow during static handgrip exercise in healthy young humans. Am J Physiol Regul Integr Comp Physiol, 301(1), R193-R200. doi 10.1152/ajpregu.00562.2010
Kennet, J., Hardaker, N., Hobbs, S., & Selfe, J. (2007).
Cooling efficiency of 4 common cryotherapeutic agents. J Athl Train, 42(3), 343-348. Retrieved from PM: 18059988
Kneale, B. J., Chowienczyk, P. J., Brett, S. E., Coltart, D.
J., & Ritter, J. M. (2000). Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol, 36(4), 1233-1238. doi:10.1016/s0735-1097(00)00849-4
Kolo, L. L., Westfall, T. C., & Macarthur, H. (2004).
Nitric oxide decreases the biological activity of norepinephrine resulting in altered vascular tone in the rat mesenteric arterial bed. Am J Physiol Heart Circ Physiol, 286(1), H296-303. doi:10.1152/ajpheart.00668.2003
Lathers, C. M., & Charles, J. B. (1993). Use of lower
body negative pressure to counter symptoms of orthostatic intolerance in patients, bed rest subjects, and astronauts. J Clin Pharmacol, 33(11), 1071-1085. doi: 10.1002/j.1552-4604.1993.tb01944.x
Liang, Y. L., Teede, H., Kotsopoulos, D., Shiel, L.,
Cameron, J. D., Dart, A. M., & McGrath, B. P. (1998). Non-invasive measurements of arterial structure and function: repeatability, interrelationships and trial sample size. Clin Sci (Lond), 95(6), 669-679. doi: 10.1042/cs0950669
Lightfoot, J. T., & Tsintgiras, K. M. (1995).
Quantification of tolerance to lower body negative pressure in a healthy population. Med Sci Sports Exerc, 27(5), 697-706. Retrieved from PM: 7674874
Lind, L., Johansson, K., & Hall, J. (2002). The effects of
mental stress and the cold pressure test on flow-mediated vasodilation. Blood Press, 11(1), 22-27. doi: 10.1080/080370502753543927
Montgomery, L. D., Kirk, P. J., Payne, P. A., Gerber, R.
L., Newton, S. D., & Williams, B. A. (1977). Cardiovascular responses of men and women to lower body negative pressure. Aviat Space Environ Med, 48(2), 138-145. Retrieved from PM:871283
Newcomer, S. C., Leuenberger, U. A., Hogeman, C. S.,
Handly, B. D., & Proctor, D. N. (2004). Different vasodilator responses of human arms and legs. J Physiol, 556(Pt 3), 1001-1011. doi:10.1113/jphysiol.2003.059717
Newcomer, S. C., Sauder, C. L., Kuipers, N. T., Laughlin,
M. H., & Ray, C. A. (2008). Effects of posture on shear rates in human brachial and superficial femoral arteries. Am J Physiol Heart Circ Physiol, 294(4), H1833-1839. doi:10.1152/ajpheart.01108.2007
Nishiyama, S. K., Walter Wray, D., Berkstresser, K.,
Ramaswamy, M., & Richardson, R. S. (2007). Limb-specific differences in flow-mediated dilation: the role of shear rate. J Appl Physiol, 103(3), 843-851. doi:10.1152/japplphysiol.00273.2007
Padilla, J., Sheldon, R. D., Sitar, D. M., & Newcomer, S.
C. (2009). Impact of acute exposure to increased hydrostatic pressure and reduced shear rate on conduit artery endothelial function: a limb-specific response. Am J Physiol Heart Circ Physiol, 297(3), H1103-1108. doi:10.1152/ajpheart.00167.2009
Parker, B. A., Ridout, S. J., & Proctor, D. N. (2006). Age
and flow-mediated dilation: a comparison of dilatory responsiveness in the brachial and popliteal arteries. Am J Physiol Heart Circ Physiol, 291(6), H3043-H3049. doi: 10.1152/ajpheart.00190.2006
Parker, B. A., Smithmyer, S. L., Jarvis, S. S., Ridout, S. J., Pawelczyk, J. A., & Proctor, D. N. (2007). Evidence for reduced sympatholysis in leg resistance vasculature of healthy older women. Am J Physiol Heart Circ Physiol, 292(2), H1148-H1156. doi: 10.1152/ajpheart.00729.2006
Platts, S. H., Martin, D. S., Stenger, M. B., Perez, S. A.,
Ribeiro, L. C., Summers, R., & Meck, J. V. (2009). Cardiovascular adaptations to long-duration head-down bed rest. Aviat Space Environ Med, 80(5 Suppl), A29-36. doi: 10.3357/asem.br03.2009
Schroeder, S., Enderle, M. D., Baumbach, A., Ossen,
R., Herdeg, C., Kuettner, A., & Karsch, K. R. (2000). Influence of vessel size, age and body mass index on the flow-mediated dilatation (FMD%) of the brachial artery. Int J Cardiol, 76(2-3), 219-225. doi: 10.1016/s0167-5273(00)00381-8
Spaak, J., Montmerle, S., Sundblad, P., & Linnarsson,
D. (2005). Long-term bed rest-induced reductions in stroke volume during rest and exercise: cardiac dysfunction vs. volume depletion. J Appl Physiol, 98(2), 648-654. doi:10.1152/japplphysiol.01332.2003
Stewart, J. M. (2002). Pooling in chronic orthostatic
intolerance: arterial vasoconstrictive but not venous compliance defects. Circulation, 105(19), 2274-2281. doi: 10.1161/01.cir.0000016348.55378.c4
Takase, B., Akima, T., Uehata, A., Katushika, S.,
Isojima, K., Satomura, K., Ohsuzu, F., Kurita, A. (2000). Endothelial function and peripheral vasomotion in the brachial artery in neurally mediated syncope. Clin Cardiol, 23(11), 820-824. doi:10.1002/clc.4960231131
Thijssen, D. H., Black, M. A., Pyke, K. E., Padilla, J.,
Atkinson, G., Harris, R. A., Parker, Blk Widlansky M. E., Tschakovsky, M. E., Green, D. J. (2011). Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol, 300(1), H2-12. doi:10.1152/ajpheart.00471.2010
Thijssen, D. H., de Groot, P., Kooijman, M., Smits, P.,
& Hopman, M. T. (2006). Sympathetic nervous system contributes to the age-related impairment of flow-mediated dilation of the superficial femoral artery. Am J Physiol Heart Circ Physiol, 291(6), H3122-3129. doi:10.1152/ajpheart.00240.2006
Thijssen, D. H., Rowley, N., Padilla, J., Simmons, G. H.,
Laughlin, M. H., Whyte, G., Cable, N. T., Green, D. J. (2011b). Relationship between upper and lower limb conduit artery vasodilator function in humans. J Appl Physiol, 111(1), 244-250. doi:10.1152/japplphysiol.00290.2011
Thomas, K. N., Cotter, J. D., Galvin, S. D., Williams, M.
J., Willie, C. K., & Ainslie, P. N. (2009). Initial orthostatic hypotension is unrelated to orthostatic tolerance in healthy young subjects. J Appl Physiol, 107(2), 506-517. doi:10.1152/japplphysiol.91650.2008
van Duijnhoven, N. T., Green, D. J., Felsenberg, D.,
Belavy, D. L., Hopman, M. T., & Thijssen, D. H. (2010). Impact of bed rest on conduit artery remodeling: effect of exercise countermeasures. Hypertension, 56(2), 240-246. doi:10.1161/HYPERTENSIONAHA.110.152868
Vaziri, N. D., Ding, Y., Sangha, D. S., & Purdy, R. E.
(2000). Upregulation of NOS by simulated microgravity, potential cause of orthostatic intolerance. J Appl Physiol, 89(1), 338-344. doi:10.1152/jappl.2000.89.1.338
Waters, W. W., Ziegler, M. G., & Meck, J. V. (2002).
Postspaceflight orthostatic hypotension occurs mostly in women and is predicted by low vascular resistance. J Appl Physiol, 92(2), 586-594. doi: 10.1152/japplphysiol.00544.2001
Wenner, M. M., Haddadin, A. S., Taylor, H. S., &
Stachenfeld, N. S. (2013). Mechanisms contributing to low orthostatic tolerance in women: the influence of oestradiol. J Physiol, 591(9), 2345-2355. doi:10.1113/jphysiol.2012.247882
White, A. R., Ryoo, S., Bugaj, L., Attarzadeh, D. O.,
Thiyagarajan, S., Chen, K., Attwater, S., Abbot, B., Li, D., Champion, H. C., Shoukas, A. A., Nyhan, D., Hare, J. M., Berkowitz, D. E., Tuday, E. C. (2010). Early changes in vasoreactivity after simulated microgravity are due to an upregulation of the endothelium-dependent nitric oxide/cGMP pathway. Eur J Appl Physiol, 110(2), 395-404. doi:10.1007/s00421-010-1514-7
*Address correspondence to:
Sara Jarvis, PhD
Department of Biological Sciences
Northern Arizona University
PO Box 5640
Flagstaff, AZ 86011
Email: sara.jarvis@nau.edu